Cobalt in Batteries
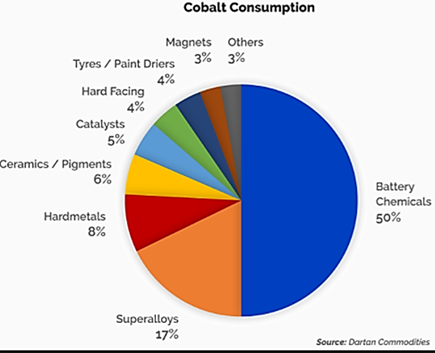
- The battery industry currently uses 50 percent of global cobalt production, a critical metal for Lithium-ion cells.
- The remaining 50 percent is used in diverse industrial and military applications (super alloys, catalysts, magnets, pigments…) that rely exclusively on the material.
- Currently the most popular technology of the rechargeable battery sector is that of the lithium-ion rechargeable batteries, also referred to as secondary batteries, a vital technology for a sustainable future.
- Whilst traditional batteries are based on galvanic action, Lithium ion secondary battery depends on an "intercalation" mechanism. This involves the insertion of lithium ions into the crystalline lattice of the host electrode without changing its crystal structure. These electrodes have two key properties. One is the open crystal structure, which allow the insertion or extraction of lithium ions and the second is the ability to accept compensating electrons at the same time. Such electrodes are called intercalation hosts.
- A typical Li-ion battery with lithium cobaltite as the cathode (LCO) can store 150 watt-hours of electricity in 1 kilogram of battery as compared to lead acid batteries, which can only store 25 watt-hours of electricity in one kilogram of battery weight.
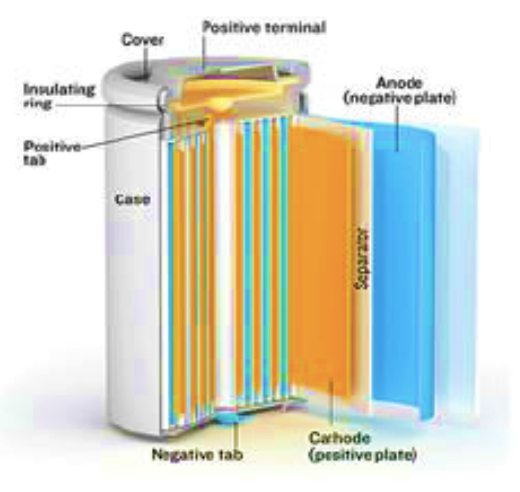
1. Battery Structure
- Positive Electrode (Cathode) = Li-Metal-Oxide, Lithium Cobaltite
- Metal typically cobalt +/-other metals
- Negative Electrode (Anode) = Graphite (Carbon)
- Electrolyte (Li Salt)
2. Battery Function
During the charge and discharge processes, lithium ions are inserted or extracted from interstitial space between atomic layers within the active material of the battery. Simply, the Li-ion is transfers between anode and cathode through lithium Electrolyte. Since neither the anode nor the cathode materials essentially change, the operation is safer than that of a Lithium metal battery.
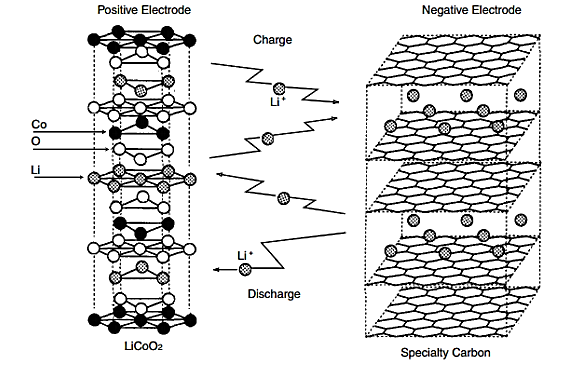
3. Principles of Working of the Li- ion Batteries:
- Compared to other battery types, Cobalt Lithium-Ion batteries deliver greatest Energy Density for Power, Performance and Charge Life. It is these qualities which are important for their utilization in electric and hybrid vehicles (EV) as well as energy storage systems, used in renewable energy farms.
- Three types are the most relevant for EV and general energy storage applications:
- Lithium-Cobalt Oxide (LCO)
- Lithium-Nickel-Manganese-Cobalt Oxide (NMC)
- Lithium-Nickel-Cobalt-Aluminum-Oxide (NCA)
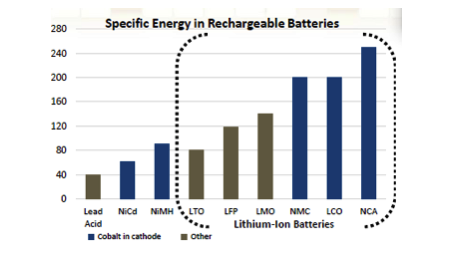
- Lithium-ion batteries containing cobalt in the cathode have superior energy, power density and superior cycling ability. Although NiMH batteries have been used within electronic vehicles in the past, the higher energy density of lithium-ion batteries makes them first choice for the manufacture of new electronic vehicles.
- Rechargeable batteries are electro-chemical devices that convert chemical energy into electrical energy.
- During discharge, electrons move from the negative anode to the positive cathode, providing a source of power. The battery is recharged by replenishing the anode with electrons to its reduced state, as well as removing the electrons from the cathode returning it to its oxidized state. The cathode structure is a matrix, often formed of cobalt oxide, which provides support for Ni/Li cations.
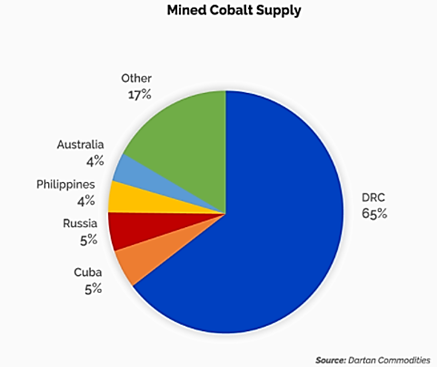
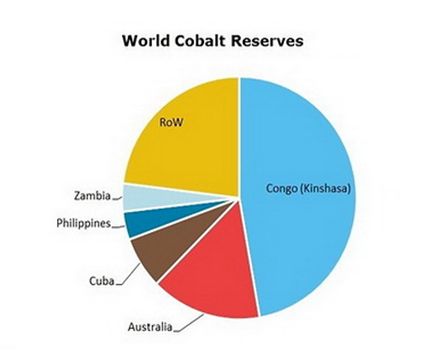
- The large role cobalt plays in cathode technology is, in part, why the EU and the USA class cobalt as a critical raw material.